Unit 1
Unit 1
Unit 1
Thermodynamics
Question 1) Explain macroscopic and microscopic approach.
Answer 1:
MACROSCOPIC APPROACH | MICROSCOPIC APPROACH |
In this approach, a certain quantity of matter is considered without taking into account the events occurring at the molecular level. | The matter is considered to be comprised of a large number of tiny particles known as molecules, which move randomly in a disordered fashion. The effect of molecular motion is considered. |
An analysis is concerned with the overall behaviour of the system. | Knowledge of the structure of matter is essential in analysing the behaviour of the system. |
This approach is used in the study of classical thermodynamics. | This approach is used in the study of statistical thermodynamics. |
A few properties are required to describe the system. | Large numbers of variables are required to describe the system. |
The properties like pressure, temperature, etc. needed to describe the system, can be easily measured. | The properties like velocity, momentum, kinetic energy, etc. needed to describe the system, cannot be measured easily. |
The properties of the system are their average values. | The properties are defined for each molecule individually. |
This approach requires simple mathematical formulas for analysing the system. | No. Of molecules is very large so it requires an advanced statistical and mathematical method to explain any change in the system |
Question 2) Explain the difference between work and heat.
Answer:
Work Heat
Interaction | Mechanical | Thermal |
Requires | Force and Displacement | Temperature difference |
Process | Macroscopic pushes and pulls | Microscopic collisions |
Positive value | W > 0 when a gas is compressed. Energy is transferred into system. | Q > 0 when the environment is at a higher temperature than the system. Energy is transferred into system. |
Negative value | W < 0 when a gas expands. Energy is transferred out of system. | Q < 0 when the system is at a higher temperature than the environment. Energy is transferred out of system. |
Equilibrium | A system is in mechanical equilibrium when there is no net force or torque on it. | A system is in thermal equilibrium when it is at the same temperature as the environment. |
Question 3) Explain the limitation of first law of thermodynamics.
Answer:
Limitations of First Law of Thermodynamics
i. The limitation of the first law of thermodynamics is that it does not say anything
About the direction of flow of heat.
Ii. It does not say anything whether the process is a spontaneous process or not.
Iii. The reverse process is not possible. In actual practice, the heat doesn’t convert completely into work. If it would have been possible to convert the Whole heat into work, then we could drive ships across the ocean by extracting Heat from the water of the ocean.
Question 4) what is thermodynamics process?
Answer:
Thermodynamics process represents a transition in which a system changes from one state to another. When the path is completely specified then the change of state is called a process. A Process is defined as the transformation of the system from one fixed state to another fixed state .When any one of the properties changes, the working substance or system is said to have undergone a process.
Some of the processes are identified by special names as given below:
i. Isobaric process (constant pressure process.
Ii. Isochoric process (constant volume process)
Iii. Isothermal process (constant temperature process)
Iv. Isentropic process (constant entropy process)
v. Adiabatic process (perfectly insulated process)
Question 5) Explain the first law of thermodynamics.
Answer:
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
Where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
Question 6) What is intensive property and extensive property ?
Answer:
Intensive property: If the property is independent of mass of the system is called an intensive property.
Example - Pressure, temperature, density, velocity, height, viscosity are the example of intensive property.
Extensive property: If the property is proportional to the mass of the system it is called an extensive property.
Example- volume, surface area, potential energy, kinetic energy, internal energy, electric charge.
Question 7) Explain thermodynamics equilibrium.
Answer: A system is said to be in the states of thermodynamics equilibrium if the value of the property is the same at all the points in the system. Or the system is said to exist in thermodynamics equilibrium when no change in any macroscopic property is registered, if the system is isolated from its surrounding.
The system is said to be in thermodynamic equilibrium if the conditions for following three equilibrium is satisfied:
1. Mechanical equilibrium
2. Chemical equilibrium
3. Thermal equilibrium
Question 8) Explain Zeroth law of thermodynamics.
Answer:
If two systems are each in thermal equilibrium with the third system then the two systems is in thermal equilibrium which each other.
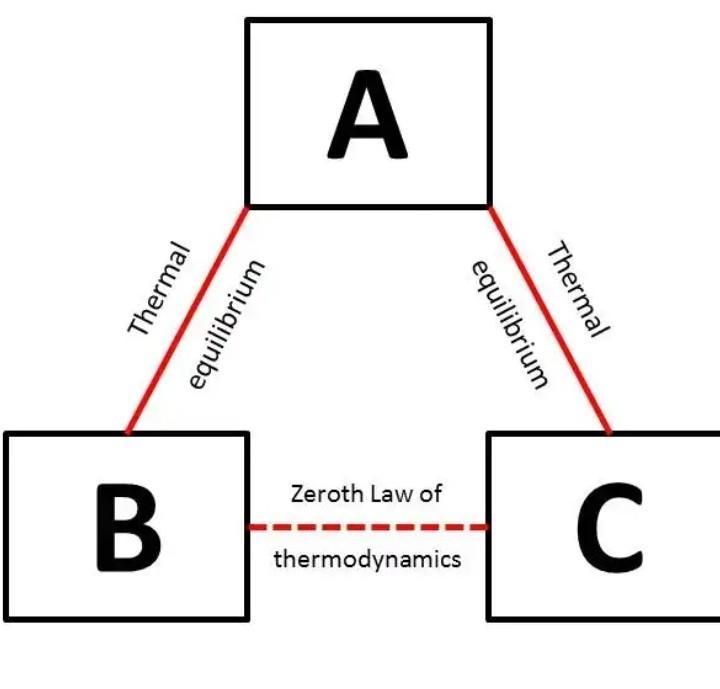
When a body A is in thermal equilibrium with a body B , and also separately with a body C ,then B and C will thermal equilibrium with each other .this is known as the Zeroth law of thermodynamics.
Question 9) Apparatus that liquefies helium is in a laboratory at 296 K. The helium in the apparatus is at 4.0 K. If 150 mJ of heat is transferred from the helium, find the minimum amount of heat delivered to the laboratory?
Answer:
To obtain the minimum amount of heat delivered to the laboratory,
Given data
150 mJ = QL,
296 K = TH
4.0 K = TL
Put these value in below formula
QH = QL (TH/ TL),
QH = QL (TH/ TL)
= (150 mj x 10-3 J/1 mJ ) x (296 K / 4.0 K)
= 11 J
From the above observation we conclude that, the minimum amount of heat delivered to the laboratory would be 11 J.
Question 10) A gas in a closed container is heated with 10J of energy, causing the lid of the container to rise 2m with 3N of force. What is the total change in energy of the system?
Answer:
Use the first law of thermodynamics.
The change in energy equals the increase in heat energy minus the work done.
ΔU=Q−W
We can find the value of work using the force and distance.
We know that Work is the product of force and displacement.
W=F x Δx
W=3N x 2m
W=6J
The total change in energy
ΔU=Q−W
ΔU=10J−6J
ΔU=4J